The FDA has recently approved the Magnetom Terra 7-Tesla, or 7T, MRI machine for diagnostic use. Magnetic Resonance Imaging (MRI) machines have been used as diagnostic tools since 1977 when the first case of cancer was identified using the device. Since then, the machines have been prescribed for use by internists, orthopedists and many other types of physicians in order to detect internal injuries or abnormalities, particularly in the organs, blood vessels, and lymph nodes. MRI results can also be used to monitor the progression of the injury or disease by taking images at regular intervals and comparing them against one another.
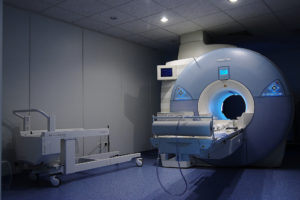
The 7T MRI machine, like its predecessors, will be used by radiologists and physicians to determine the presence of internal injuries or disease. MRI machines impose a magnetic field on a specific area of the patient’s body, which then causes the protons located in the water molecules inside the body to align and spin. A radio frequency, which causes the strength of the magnetic field to fluctuate, is also emitted, changing the patterns of the spinning protons. These two signals help to produce images that the technician can capture and pass along to the radiologist for interpretation. This device and procedure allow physicians to diagnosis internal maladies through non-invasive means.
MRI machines create magnetic fields in various strengths which are measured in Tesla (T) units. The strength of the field will be determined by the intensity of the magnet used; the stronger the magnet, the higher the Tesla unit value. A magnet measuring one Tesla unit is about 1,000 times the strength of a typical refrigerator magnet. The standard MRI magnet measures 1.5T, with the previous strongest MRI magnet measuring 3T. The newest 7T machine has a magnet that is more than twice as strong as previous MRI magnets, and 7,000 times stronger than standard magnets.
While the 3T model previously provided the most elaborate imaging, the 7T MRI device has a significantly higher sensitivity than any other models. The magnetic field created by the machine is much stronger, leading to clearer and more detailed images. The 7T machine also produces large data sets, providing over 7,000 images from one diagnostic session. This new and improved model allows physicians to diagnose and treat their patients with increased efficiency and accuracy.
Though the strength of the magnet may sound concerning, the FDA has studied the machine and the safety of the radio-frequency waves emitted. Comparative data from the 3T and 7T models, as well as computer modeling and simulations with the 7T MRI machine, were evaluated by board-certified radiologists. These studies indicated that the machine was an accurate and often superior diagnostic tool, and is safe for most individuals requiring medical imaging.
In addition to its safety and diagnostic benefits, the 7T MRI machine features an improved workflow for hospitals or testing centers. The magnet itself is significantly lighter than previous models and can be easily shipped and installed. The Magnetom Terra also features advanced imaging technology that makes it easier for technicians to capture high-quality diagnostic images.
However, there are some limitations to the use of the Magnetom Terra 7-Tesla MRI machine. The 7T magnet should only be used on people weighing more than 66 pounds. Additionally, it has only been approved for imaging of the extremities. The 7T model should only be used for diagnoses involving the head, arms, and legs.
The approval of the 7T MRI machine will allow physicians and radiologists to provide more comprehensive assessments and results for patients with potential internal injuries or disease. The more detailed images provided by the strength of the magnet in this device can lead to quicker and earlier diagnoses. This may allow patients to get treated sooner, potentially leading to better outcomes long-term.