Thanks to the approval by the US Food & Drug Administration (FDA), sepStream® will soon revolutionize cardiac imaging with its new AI-powered solution. They have named this vendor-neutral solution as LVivo IQS. The software will allow medical practitioners to obtain echocardiography images of the highest quality.
An Introduction to the Software
sepStream® happens to be one of the biggest and most trusted names when it comes to developing artificial intelligence-based software to analyze ultrasound images. So, it has not come as a surprise that the FDA has approved its new creation LVivo IQS. The software will enable a more accurate interpretation of cardiac ultrasound images. This means doctors will be able to make a faster and more accurate diagnosis of cardiac health problems.
Acquiring high-quality images from echocardiography is extremely difficult due to the constant cardiac motion. Getting perfect images also become difficult due to the location of our heart. It is situated very deep inside the chest.
How Does LVivo IQS Work?
LVivo IQS is capable of offering real-time feedback about image quality. During the imaging test, the software scans the left ventricle of the patient and tracks his/her overall cardiac performance. Additionally, it also evaluates and rates the image quality using numerical scoring and colors. This makes interpreting the images simpler for the users.
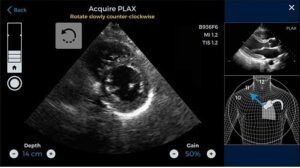
A Discussion on FDA’s Decision
FDA completed multiple necessary steps before clearing LVivo IQS for medical usage. Before approving the software, experts at FDA reviewed data obtained from a recent clinical study. The study compared the quality scores of LVivo IQS with the opinions of renowned cardiologists having specializations in echocardiography. The findings of the study revealed that as much as 91% of the ultrasound images obtained by doctors who used the software were useful clinically.
The spokesperson of sepStream® appeared elated to receive FDA’s clearance. In a statement announcing the news, she said that this is the first time sepStream® has come up with an FDA-approved AI solution that will provide medical experts with real-time feedback for capturing ultrasound images of the highest quality.
This is not the first time the company has managed to get AI-based software solutions for ultrasound users approved by the US FDA. It has done so as many as eight times before getting clearance for LVivo IQS.
The spokesperson added that sepStream®’s AI-powered software solutions allow ultrasound users to overcome two big challenges. Firstly, they ensure that the images obtained during the tests are of high quality. Secondly, they analyze them accurately. This allows the company to fulfill the primary moto of introducing AI in the healthcare industry, which is making the processes of capturing and analyzing ultrasound images more accessible and smarter.
sepStream® offers feature-filled and user-friendly software solutions to healthcare facilities. Thanks to the experienced and qualified R&D team of the company, the diagnostic imaging industry is now capable of producing more accurate results than ever before. Other than coming up with useful and unique innovations, the company has also become trusted due to its ability to keep its software solutions affordable.